Abstract
Hematopoietic stem cell (HSC)-mediated gene therapy or editing holds tremendous promise for many genetic diseases, HIV and cancer. Currently, most if not all approaches focus on the gene modification of CD34+ cells. However, CD34+enriched cell products predominantly contain lineage-committed progenitors and only very few "true" long-term engrafting HSCs which are ultimately the cells needed for sustained correction of diseases. Here we have evaluated a novel HSC-enriched phenotype for HSC transplantation and gene therapy.
Most recently reported mouse xenograft transplants of purified and gene-modified human CD34+CD38- HSCs led to delayed myeloid engraftment suggesting the loss of crucial short-term engrafting progenitor cells. With the goal to identify a more refined HSC-enriched target cell population for transplantation and gene therapy, we recently performed comprehensive in vivo studies of CD34 subpopulations in our nonhuman primate (NHP) stem cell transplantation model. Comparing lentivirus (LV) gene-modified candidate cell fractions in competitive reconstitution experiments, we found an HSC-enriched phenotype (CD34+CD45RA-CD90+) demonstrating both rapid short-term recovery as well as robust long-term engraftment after myeloablative conditioning and autologous transplantation. Surprisingly and in contrast to published engraftment studies of equivalent human CD34 subpopulations in the mouse xenograft model, cell fractions enriched for multipotent progenitor cells (MPPs, CD34+CD45RA-CD90-) or lympho-myeloid progenitors (LMPs, CD34+CD45RA+CD90-) did not measurably contribute to short-term hematopoietic reconstitution in the NHP model.
To determine whether the observed lack of MPP/LMP-contribution in the NHP may have been caused by ex vivo culture and/or LV gene modification conditions, engraftment studies of freshly isolated and sort-purified NHP CD34 subpopulations were performed in a novel "monkeynized" mouse model. Similar to our studies in the autologous setting, multi-lineage engraftment was exclusively driven by CD34+CD45RA-CD90+ cell fractions even freshly-isolated and transplanted without co-administration of MPPs/LMPs. Maturation of all blood lineages was found in the peripheral blood (PB), bone marrow (BM), spleen and thymus of successfully engrafted mice. Furthermore, NHP CD34+CD45RA-CD90+ cells were exclusively capable of homing into and repopulating the BM stem cell niche giving rise to phenotypical MPPs, LMPs as well as downstream progenitors.
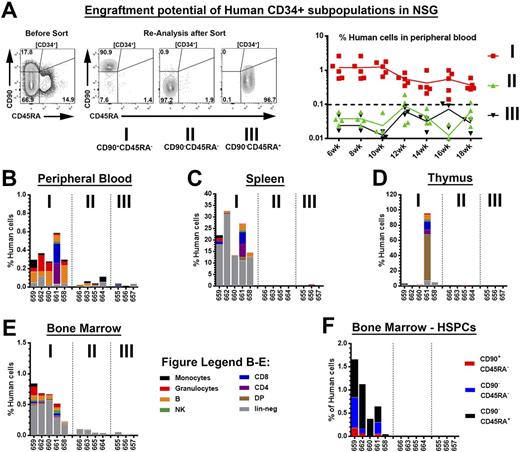
We next performed comprehensive mouse xenograft studies of freshly isolated and phenotypically identical human CD34+ subpopulations (Figure 1A) to evaluate this HSC-enrichment strategy with human stem cell sources. Similar to our studies with NHP HSPCs, multi-lineage engraftment in PB, BM, spleen and thymus was primarily restricted to CD34+CD45RA-CD90+ cell fractions (Figure 1A-E). Furthermore, engraftment and repopulation of the BM stem cell compartment was only observed for transplanted CD34+CD45RA-CD90+ HSPCs (Figure 1F). Surprisingly, we did not see engraftment of transplanted MPP- and LMP-enriched human cell fractions.
In summary, these data demonstrate and confirm that both human and NHP CD34+CD45RA-CD90+ HSPCs are primarily responsible for multilineage hematopoietic reconstitution. In contrast to other HSC-enrichment strategies, the identified phenotype contains both short-term as well as long-term engraftment potential demonstrating no measurable delay in the onset of hematopoietic recovery upon transplantation in the NHP model. Most importantly, this phenotype is maintained throughout ex vivo culture and/or gene-modification, reduces the number of cells for gene-modification 20 to 30-fold, and likely increases targeting/editing efficiency as well as safety, making it a highly attractive target cell population for HSC-mediated gene and cell therapy approaches.
Figure Legend - Multi-lineage engraftment potential of human CD34+ subpopulations in NSG mice: (A) Human CD34 subpopulations from GCSF-mobilized apheresis products were sort-purified and injected intravenously into sub-lethally irradiated adult NSG mice (left). Frequency of human cells in the PB over time in transplanted NSG mice (right). (B-E) Multi-lineage engraftment of human cells in the (B) PB, (C) spleen, (D) thymus and (E) BM. (F) Engraftment of human HSPCs in the murine BM.
Adair: CalImmune Inc.: Consultancy; Rocket Pharmaceuticals: Consultancy, Equity Ownership, Patents & Royalties, Research Funding. Kiem: Rocket Pharmaceuticals: Consultancy, Equity Ownership, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.